Atomic Number of Elements Define Which of the Following
Element is O s with atomic no 7 6. Sum of number of protons and number of neutrons present in the nucleus.

Nobelium Chemical Element Britannica
The number of protons in the atomic nucleus.

. Atoms of an element having d. In the periodic table the vertical columns are called groups and the horizontal rows are called periods. Number of protons of an atom or nuber of electrons of a neutral atom.
B Atomic WeightAtomic Mass Number. The symbol for cerium in Ce. In a tabular format list the symbol mass number charge and composition of alpha particle beta particle and gamma rays.
It belongs to metalsCerium with atomic number of 58. What four things are shown on a periodic table square for an individual element. Species having same atomic number but different mass number.
List of the Elements. The valency of an element is determined by the number of valence electrons present in the atom of the element. Define the following terms.
Additionally how many electrons protons and neutrons are there in a nucleus of atomic number 11 and mass number 24. Below the periodic table in the third paragraph click on how to calculate the number of protons neutrons and electrons in an atom of an element 5. Atomic number of carbon 6 Atomic number of nitrogen 7 Atomic number of oxygen 8 Atomic number of magnesium 12.
Iron with 30 neutrons 56 4. B Mass number. Gallium with 39 neutrons 70 d.
4 Atomic mass number. A protons plus neutrons in its nucleus. The number of electrons lost or gained or shared by one atom of an element to achieve the nearest.
Get Instant Solutions 24x7. Answer verified by Toppr. The number of protons define the identity of an element ie an element with 6 protons is a carbon atom no matter how many neutrons may be present.
An elements identity is defined by its atomic number. It belongs to metals. There are 118 elements on the periodic table.
For example Silicon has an atomic number of 14 so it has 14 protons in the nucleus. Atomic number is also equal to numbers of electrons in an atom. The atomic number of a chemical element is the number of protons in the nucleus of an atom of the element.
Accordingly the number of protons which is always equal to the number of electrons in the neutral atom is also the Atomic Number. Determines the chemical properties of an element along with its place in the periodic table The number of protons in the nucleus of an atom. Give the mass number of each of the following atoms.
The number of protons define the identity of an element ie an element with 6 protons is a carbon atom no matter how many neutrons may be present. Number of electrons present in an atom which is equal to number of protons in a neutral atom. 16 The following data represents the distribution of electrons protons and neutro elements A B C D.
This number is the atomic number. Writing and balancing equations of Nuclear Reaction. A Atomic number is the number of protons in the nucleus of the atom.
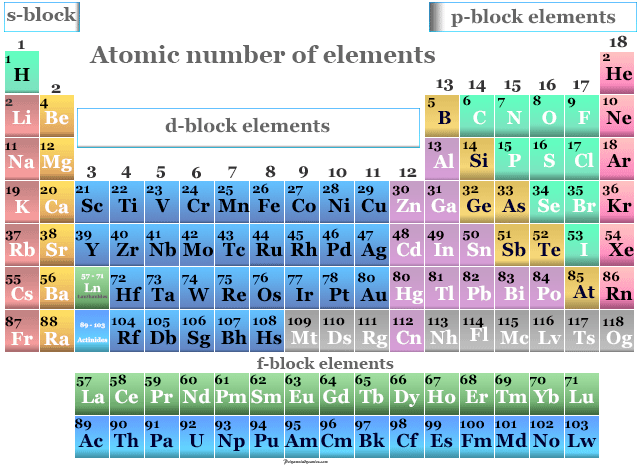
Periodic Table of Elements - The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. The atomic number determines the identity of an element and many of its chemical properties. A Atomic number.
Consider the following elements. Define the atomic number of element which is present just below the R u. B electrons in the element.
Updated on September 09 2019. D Binding energy is theamount of energy that holds. B Atomic mass or Mass number is the total number of protons and neutrons nucleonsin the nucleus of an atom.
In the modern periodic table the elements are listed in order of increasing atomic number. The atomic number refers to the number of a chemical element in the periodic system whereby the elements are arranged in order of increasing number of protons in the nucleus. A chemical elements atomic number is the number of protons in.
For example in a sodium atom there are 11 electrons and 11 protons. Atom is the structural unit of the matter and smallest component of an element. 119 rows Define Atomic Number.
The number of protons present in an atom is called its atomic number. Valency- The combining capacity of an element is called its valency. It is represented by the symbol.
7 Define the following terms. 2 Element are those substance which can not be split into simpler substancethey are made up of single atom. Atomic number Number of protons.
C Isotopes are the elements that have the same atomic number but have different mass numbers. It is the charge number of the nucleus since neutrons carry no net electrical charge. Determines the chemical properties of an element along with it s place in the periodic table.
Each element is identified by the number of protons in its atoms. Draw and label a sample element. Define each of the following A Atomic number The number of protons in the nucleus of an atom.
The number of protons in the nucleus of an atom which is characteristic of a chemical element and determines its place in the periodic table. Thus the atomic number of Na atom number of electrons number of protons 11. Atomic mass is the sum of the total number of protons and neutrons present in the nucleus of an atom.
The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. Visit BYJUS to learn Symbols Atomic Number Atomic Mass Groups Videos with FAQs of Periodic Table Elements. The periodic table lists the elements in order of increasing atomic number.
Upvote 0 Was this answer helpful. Define the following terms a. Which of the following best describes an atomic number.
We review their content and use your feedback to keep the quality high. I Valency ii Atomic size. The atomic number is the number of protons in the nucleus of an atom.
The atomic number of an element represents the number of protons that are in the nucleus. The smallest part into which an element can be divided. 5 otons and neutrons in atoms of foll Q.
So for your question the Periodic Table tells us that sodium has an Atomic. Atomic Number 22 ProtonsElectrons 22 Neutrons - 26 3. Titanium with 26 neutrons - 48 c.
Sum of the number of protons and neutrons ie. Beryllium with 5 neutrons - 9 b. Answer Explanation1 Explanation.
Each element has a symbol which is one or two letters. Isobars are the atoms of different elements having a different atomic number but the same mass number. This means it represents the number of options.
Thorium element number 90.

What Is An Atomic Number Definition And Examples
Atomic Number And Atomic Mass Number Definition Examples Questions
No comments for "Atomic Number of Elements Define Which of the Following"
Post a Comment